|
Antibiotics Resistance of Bacterial
Biofilms

Amna
Butt
Aisha Khan
Fatima
Jinnah Women University
Rawalpindi
Pakistan
Corresponding author:
Aisha Khan
Fatima Jinnah Women University,
the mall,
Rawalpindi
Pakistan
Email:
aishahayat85@yahoo.com

Abstract
Biofilms are microorganism communities
that get attached to a surface. It
is evident through various research
that cells grown in a biofilm express
distinct properties from that of planktonic
cells. One such property which is
of high significance is an increased
resistance to antimicrobial/ antibiotic
agents. Bacterial biofilms are a cause
of chronic infections worldwide because
of their increased antibiotic tolerance.
Their resistance to the antibiotics
is due to mutation, resistant phenotypes,
adaptations to stress, quorum sensing,
stratified activity, nutrient gradients,
oxidative stress, failure of antibiotic
penetration and heterogeneity. Thus
in order to control the bacterial
infections caused by these biofilms,
there is a need of novel drug delivery
approaches and enhanced therapeutic
use of quorum sensing inhibitors.
Key words: Biofilms, antibiotic
resistance, quorum sensing, quorum
sensing inhibitors

1. Introduction
A biofilm is a complex structure that
adheres to surfaces regularly in contact
with water. Microorganisms secreting
mucilaginous protective coating in
which they are encased usually form
biofilms. Generally colonies of bacteria
and other microorganisms such as fungi,
yeasts and protozoa form biofilms.
Biofilms generally form on liquid
or solid surfaces in addition to soft
tissues in living organisms. Thus
they show aspects of both liquids
and solids (much similar to slug slime)
and come under a category named "viscoelastic".
Conversely, as biofilms become scaled
with calcium deposits or rust or collect
sediment they become more like a brittle
solid and less fluid. Typically biofilms
show considerable resistance to conventional
disinfection methods. Examples of
biofilms include dental plaque, algal
mats on water bodies and the slimy
coating that fouls pipes and tanks.
Van Leeuwenhoek was the first to observe
microorganisms on tooth surfaces by
making use of his simple microscopes
and thus was the one who made the
discovery of microbial biofilms. Over
the years several other scientists
studied biofilms including H. Heukelekian
and A. Heller in 1940 and C. E. Zobell
in 1943 however detailed assessment
of biofilms had to await the development
of the electron microscope, which
permitted high-resolution photo-microscopy
at magnifications that were much higher
than that of light microscope (Donlan,
2002).
Biofilms are extremely heterogeneous
in nature. Several observations and
measurements on various biofilms have
been made; all of them point to the
diversity found in individual biofilm
colonies. Typically, naturally occurring
biofilms almost always have a quantitatively
large number of different kinds of
organisms living in colonial form.
Additionally, different biofilms seemingly
show different electrical properties,
chemical properties, internal structures,
and, definitely, different properties
of pretty nearly any other observation
that can be made. These properties
contribute to differential characteristics
of the biofilm (e.g., hard to kill)
compared to individual microorganisms
in isolation (in a planktonic environment)
(Cunningham, Lennox and Ross, 2008).
1.1. Biofilms Formation Process
Generally development of a biofilm
occurs in different chronological
phases, which are illustrated in Figure
1 below:
Figure 1. Major steps involved
in biofilm formation (Source: Harrison,
2007)
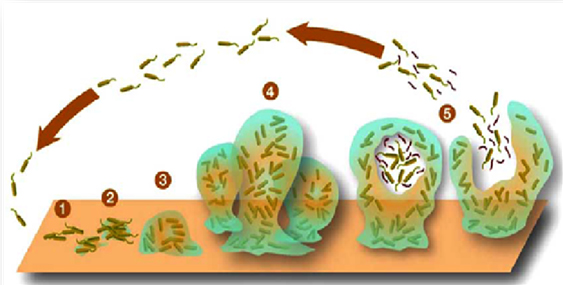
1.1.1. Surface conditioning
Surfaces on which biofilms are attached
are first conditioned by adsorption
of inorganic and organic nutrients.
This adsorption is required to influence
the subsequent bacterial attachment.
Surface conditioning can increase
tolerance to antibiotics for example;
Pseudomonas aeruginosa biofilms developed
better tolerance to tobramycin (antibiotic
agent) and large cellular aggregates
when attached to surfaces conditioned
with the glycoprotein mucin and allowed
to grow there.
1.1.2. Reversible attachment
The preliminary transport of bacterial
cells and their reversible attachment
to a surface usually occurs by brownian
motion of the cells, active movement
of motile bacteria, physical and electrostatic
interactions between the cell surface,
convection currents within a bulk
liquid responsible for transporting
bacteria to the surface, substratum
and sedimentation. This state of bacterial
adhesion to surface may result in
symmetrical distribution between suspended
and adhering cells, and is considered
as the weakest link in the series
of events that connect the bacterial
cells to the surface conditioned beforehand
(Landry et al. 2006).
1.1.3. Irreversible attachment
Because of stimulation of membrane
bound sensory proteins, the cells
which were attached reversibly to
surface now produce extracellular
polymeric substances which allows
the cell-to-cell bridges development
that attach the cells to the surface
irreversibly.
1.1.4. Colonization
Surface colonization is the final
phase of biofilm formation. Attached
bacteria form micro-colonies by growing
and dividing. These micro-colonies
are considered as the fundamental
units of organization of a biofilm.
Other planktonic cells also get entrapped
in the extracellular polymeric substances,
resulting in the establishment of
a biofilm. Primary colonizers i.e.
one bacterium that colonizes a surface,
often influence the secondary colonizers
i.e. attachment of others to the same
surface.
1.1.5. Detachment
Initially the researchers suggested
that the detachment of clumps of biofilm
cells and subsequent transfer and
attachment to other surfaces might
be due to turbulent shear forces.
Such a mechanism of detachment can
only be accurate for biofilms which
grow under laminar shear forces and
are seemingly detached due to turbulent
shear forces. Nevertheless, recent
studies have suggested that detachment,
frequently termed as 'dissolution'
or 'dispersion', is an active and
highly regulated process controlled
by the attached cell populations (Lindsey
and Holy, 2006). (Figure 1)
1.2. Properties of Biofilms
Some apparent characteristics common
to almost all biofilms being observed
are as follows:
• Biofilms are responsive to
their environment and are dynamic
in nature; that is, they are very
adaptive to environmental changes.
• Detachment phenomenon is common
among all biofilms. Through this phenomenon
individual or clumps cells of bacteria
become able to detach themselves from
the biofilm colony.
• Detached individual microorganisms
are comparatively easy to kill in
isolated form with specifically designed
chemicals for this purpose (antibiotics).
• The detached microorganisms
of the biofilm are the cells in clump
form that are just not attached to
the biofilm at that time. However,
these clumps are able to maintain
the original properties of the parental
biofilm and thus are a lot harder
to kill.
• In favorable conditions, biofilms
can migrate from surface to surface
in a variety of ways over a period
of time. This migration may be via
streaming, rippling, detaching, seeding
dispersal and rolling as illustrated
below in Figure 2.
Figure 2. Biofilm migration (Source:
Cunningham, Lennox, and Ross, 2008)

• Another main characteristic
of the cells that are found in a biofilm
is their ability to communicate with
one another. Bacterial communities
communicate with each other using
different chemical signals. These
chemicals are produced and passed
by outer membranes of these cells
and can be interpreted by members
of the same cell species as well as
other microbial species present in
the same biofilm community.
• These chemical signals are
sensed by adjacent cells in the biofilm
and can induce different behavior
of neighboring cells due to occurring
of different genetic expression in
those cells (Figure 3).
Figure 3: Communication mechanism
within a Biofilm community. (Source:
Cunningham, Lennox, and Ross, 2008)
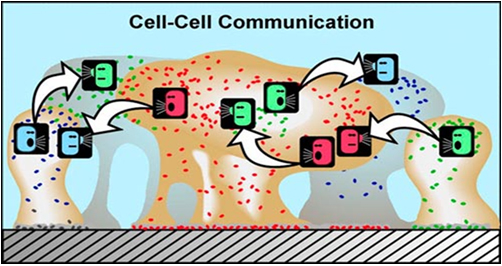
• In Figure 3, different colors
represent different bacterial species.
Bacteria can "talk" to others
and they "listen" or respond
to the chemical signals produced by
the other. This type of interaction
produces behavioral changes because
in biofilms the population is numerous
enough to initiate genetic activity.
Although the same signals are produced
in planktonic populations they are
not concentrated enough to cause genetic
expression change while passing through
water.
• There is a population recognition
system present in biofilm which is
termed as "quorum sensing".
For example, bacterial pathogens only
produce toxins when they sense that
a sufficient population is present
to survive host defenses (e.g., antibodies).
Vibrio fischeri, a marine bacterium
was the first one in which this phenomenon
was observed which produced light
after ensuring that an adequate population
of this bacterium has been developed
in biofilm.
• The coordinated behavior of
biofilm is responsible for the survival
strategies against host immune system
and antimicrobial agents.
2. Bacterial
Biofilms
Formation of bacterial biofilms occurs
when unicellular organisms join each
other to shape a community which attaches
itself to a solid surface and sheathed
in an exopolysaccharide matrix. Single
or multiple bacterial species can
make up biofilms. For instance, the
estimations reveal that dental biofilms
contain more than 500 different bacterial
taxa; on the contrary, the primary
bacterium found in latter stages of
cystic fibrosis (CF) patients' lung
is Pseudomonas aeruginosa. The bacteria
of the same kind act or behave differently
(or show different properties) when
they are in a biofilm in comparison
to their isolated or planktonic form
(that is, freely floating bacteria
as single cells in water). Some of
the very important characteristics
of bacteria growing in biofilm are
dissimilar to the planktonic bacteria
and this has noteworthy therapeutic
and diagnostic consequences. This
difference is due to the location
of bacteria in biofilm infections
in aggregates surrounded by the self-produced
matrix. Biofilms can be recognized
in clinical specimens (biopsies, septum,
pus) by making use of light microscopy,
though all the bacteria within a biofilm
cannot be identified precisely. Their
identification requires specialized
staining techniques. Additionally
the bacteria growing in a biofilm
cannot be cultured by traditional
sampling techniques unless they are
released by ultrasonic pre-treatment
(Moskowitz et al. 2004; Bjarnsholt
et al. 2007; Hoiby et al. 2010).
It has been observed that the resistance
of bacteria to an antibiotic is also
amplified in comparison to what is
seen usually with planktonic cells.
In effect, when these cells exist
in a biofilm, they utilize 10-1000
times more resistance to the effects
of antibiotic agents (Evan and Holmes,
1987; Mah and O'Toole, 2001). These
antimicrobial drugs are traditionally
developed to kill planktonic bacteria
(free bacteria) by assuming that they
would destroy the same bacterial species
irrespective of wherever or in what
form they were found. However studies
reveal that:
1. Planktonic bacteria are
more prone to antibiotic chemicals
intended to destroy them than are
the bacteria present in biofilms,
and
2. Numerous infections that
effect humans are in fact caused by
bacterial colonies present in the
biofilm state, not the bacteria in
planktonic state.
With this information along with the
fact that traditional antibiotics
have been tested and designed for
bacterial population in their planktonic
growth mode which is relatively unprotected,
we can begin to understand the basic
reason behind the antibiotics resistance
of same bacteria when they exist in
a biofilm (Cunningham, Lennox, and
Ross, 2008).
3. Antibiotic
Tolerance/Resistance Of Bacterial
Biofilms
Since the discovery of penicillin
in 1938, there has been a tremendous
success in control of acute bacterial
infections by the help of antibiotics.
Microbiologists have predicted in
vivo effects of antibiotic effects
in vitro evaluation of the minimal
bactericidal concentration (MBC) and
minimal inhibitory concentration (MIC).
MBC and MIC help in assessing the
effect of antibiotics against planktonic
bacteria in the exponential phase
of growth and consequently predict
effectiveness of antibiotic against
rapidly dividing bacteria in acute
infections quite correctly. However,
biofilms show a resistance against
these antibiotics and thus are of
major importance from a clinical point
of view as more than 60% of the infections
due to bacteria, currently treated
in the developed world by physicians
involve the bacteria responsible for
biofilm formation, some of which are
given in Table 1. This increased tolerance
of bacterial biofilms to disinfectant
chemicals and antibiotics as well
as resistance to phagocytosis and
other mechanisms of the defensce system
are a cause of many chronic infections.
For instance, persistence of staphylococcal
infections related to foreign bodies
is due to formation of biofilm. Similarly,
chronic lung infection due to Pseudomonas
aeruginosa in CF patients is caused
by biofilm-growing mucoid strains.
(Table 1)
Table 1. Human infections involving
biofilms
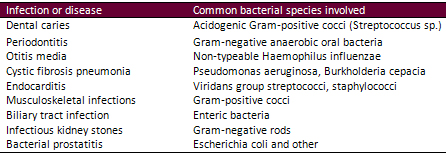
(Source: Fux et al. 2005)
Biofilms have a triple role to play
in the spread of antibiotic resistance
such as:
• The treatment of infections
caused by biofilms requires long-term
and recurrent therapy with antibiotics.
• Physiology of biofilm enables
bacteria embedded in them to survive
long term exposure of antibiotic to
acquire specific resistance to the
particular antibiotic agent.
• The accumulated mobile genetic
elements within biofilms, high cell
density and increased genetic competence
provide an ideal condition for efficient
horizontal gene transfer (Fux et al.
2005).
The major factors that are involved
in rendering antibiotic resistance
to bacterial biofilms are explained
in Figure 4 given below. (Figure 4)
Figure 4. Antibiotic resistance
of bacterial biofilms (Source: Stewart
and Costerton, 2001)
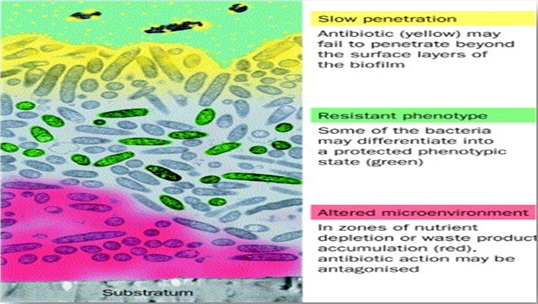
3.1. Mutation
The frequency of mutations in bacteria
growing in biofilms is significantly
higher in comparison to bacteria growing
in planktonic environment and additionally
the horizontal gene transmission is
also found to be higher in biofilms.
These physiological conditions explain
the reason behind multidrug resistance
of bacterial biofilms. These bacteria
easily become resistant to aminoglycosides,
fluoroquinolones and lactam antibiotics.
Bacterial biofilms may simultaneously
produce antibiotic degradation enzymes.
It has been shown experimentally that
mutations in bacterial biofilms establish
the surfacing of antibiotic resistance,
especially due to expression of multidrug
efflux pumps. The cause of enhanced
mutability is considered to be a result
of oxidative stress to which the bacterial
biofilms are subjected. Researchers
show that due to oxidative stress
within the biofilms, there are specific
sites within them for superior genetic
adaptation and evolutionary change.
Thus the hypermutability of bacterial
biofilms promotes the antibiotic resistance
to biofilms conferred by mutations
(Driffield et al 2008; Conibear et
al. 2009).
3.2. Presence of Resistant Phenotype
There is a biofilm-specific phenotype
which is resistant to antibiotics.
This phenotype is present within a
subpopulation of the biofilm community
which results in the expression of
active mechanisms for making the biofilm
antimicrobial resistant. Generally
when the biofilm attaches to a surface
their expression is a general phenotype
representative of that biofilm and
the subpopulation genes are repressed.
However, it is a possibility that
just a subset of these biofilm cells
could express genes for augmented
resistance to antibiotics (Mah and
O'Toole, 2001; Fux et al. 2005).
3.2.1. Induction of a Biofilm Phenotype
The resistant phenotype is maybe induced
by certain types of environmental
stress, nutrient limitation, high
cell density or an amalgamation of
all of these happenings. Another mechanism
for inducing antibiotic resistance
in biofilm cells is the membrane-protein
composition alteration in response
to exposure of antibiotic agents.
The resultant change could decrease
the permeability of the cell to these
substances (Mah and O'Toole, 2001;
Fux et al. 2005).
3.3. Bacterial Adaptation to Stress
and Damage
Another reason for antibiotic resistance
of bacterial biofilms is the ability
of bacteria to adapt itself to stress
conditions. For instance, organisms
present in a biofilm increase their
capacity to neutralize and withstand
monochloramine, induce the expression
of chromosomal betalactamases or stimulate
catalase production when subjected
to prolonged and concurrent subjection
to or treatment with the respective
antibiotic compounds. Bacterial biofilms
can switch themselves to more tolerant
phenotypes upon facing prevalent environmental
stresses such as temperature alterations,
alterations in osmolarity, pH, cell
density and nutritional quality by
turning on stress-response genes.
Stress-response genes produced by
the bacterial biofilms can not only
antagonize the deleterious effects
of antibiotics but also that of the
environmental toxins and immune system
on bacteria. These genes are regulated
by the interacting signals, i.e. quorum
sensing (Boaretti 2003).
3.4. Quorum Sensing and High Cell
Density
As mentioned earlier, bacteria in
a biofilm communicate with others
present in the same biofilm by synthesizing
and reacting on some chemical signals.
This phenomenon is referred to as
Quorum Sensing (QS) and it allows
bacteria to sense when a sufficient
amount (or concentration) of bacterial
cells are in attendance in a confined
space of biofilm environment and thus
act in response to it by certain gene
activation that results in production
of virulence factors such as toxins
or enzymes. This relationship between
biofilms and QS is termed as sociomicrobiology.
The QS molecules (signals) are peptides
of small size in many Gram-positive
bacteria, while N-acyl-l-homoserine
lactones present in Gram-negative
bacteria are the most well described
QS molecules (Jensen et al. 2007).
An example of QS's role in designating
antibiotic resistance to biofilms
is that for Pseudomonas aeruginosa;
QS is responsible for regulating the
virulence factors production such
as cellular lysins and extracellular
enzyme, which are important for the
pathogenesis of infections by functioning
as a protective shield against phagocytes.
In addition QS has also been shown
to determine the resistance or tolerance
of biofilms of Pseudomonas aeruginosa
to antibiotic therapy and to the innate
inflammatory response (Gennip et al.
2009; Alhede et al. 2009).
3.5. Stratified Activity and Low
Oxygen Concentration
Examination of environmental and in-vitro
biofilms exposed that the concentration
of oxygen and other nutrients might
be high at the surface but moving
towards the centre of the biofilm
anaerobic conditions might exist and
nutrient concentration may decrease
thus forming gradients on nutrients
and oxygen. This gradient formation
is related to the decreased metabolic
activity of bacteria and thus increases
doubling time. It is this somewhat
dormant state that is responsible
for some of antibiotic tolerance of
biofilms. This could be explained
by the fact that planktonic organisms,
though they utilize nutrients, do
not have adequate metabolic activity
required for their depletion. Thus
when they are present in biofilm,
in form of a group, their collective
metabolic activity reduces further
and leads to formation of concentration
gradients of substrates and localized
chemical microenvironments leading
to stratified protein synthesis, growth
and metabolic activity in biofilms,
i.e. a high level activity at the
surface of biofilm while a low level
activity along with slow or no-growth
persistent in the center of biofilm.
Thus stratified activity along with
low concentration of oxygen and reduced
growth rate may result in less liability
of biofilms to antibiotics or antimicrobials
(Werner et al. 2004; Hoiby et al.
2010).
3.6. Failure of Penetration of Antibiotic
in the Biofilm
The glycocalyx or exopolysaccharide
matrix production is one of the distinctive
traits of biofilms. This matrix, among
performing other functions, prevents
antibiotics' access to the bacterial
cells entrenched in the community.
Usually, it is either compound's sorption
to or its reaction with the components
of the biofilm, which limits the transportation
of antimicrobial agents into the cells
embedded in biofilm. Although it is
suggested by various mathematical
models that there should be no barrier
to their diffusion into a biofilm
for the antibiotics, some researchers
have revealed a noticeable failure
of penetration of certain antibiotic
agents to the biofilm. For instance,
as measured by a chlorine-detecting
microelectrode, Chlorine which is
a common disinfectant, failed to reach
20% of the concentration of bulk media
within a mixed Klebsiella pneumoniae
and Pseudomonas aeruginosa biofilm.
Indeed, the penetration profile suggested
that the substrate got consumed within
the matrix. Other researchers made
use of infrared spectroscopy to show
that the transportation rate of the
antibiotic ciprofloxacin to the colonized
surface was reduced in comparison
to a sterile surface. It was suggested
that the low penetration of ciprofloxacin
was due to its binding to the biofilm
components. Even the antibiotic agents
that get adsorbed into the biofilm
matrix have shown to have a retarded
penetration. Slow penetration of aminoglycoside
antibiotics is one such example. These
antibiotics are positively charged
agents that bind themselves to negatively
charged polymers in the matrix of
biofilm (Stewart and Costerton, 2001).
3.7. Heterogenity
Any bacterial cell present within
the biofilm experiences a somewhat
different environment as compared
to the neighboring cells present in
the same biofilm, and therefore has
a different growth rate. The nutrients,
signaling factor and waste products
gradients that are formed within a
biofilm allow for the heterogeneity
to develop within the biofilm. This
heterogeneity can also be one of the
reasons for the antibiotic resistance
of biofilms. For example in an experiment
when biofilm cells were subjected
to the antibiotic treatment with fleroxocin,
cell elongation occurred which was
at extreme level in exposed cells
i.e. the ones located on the uncovered
side of the biofilm. These studies
disclose that the response to antibiotics
can vary greatly, depending on the
location of a particular cell within
the community of biofilm. Thus the
distribution of bacterial survival
is irregular within seemingly identical
microenvironments.
4. Anti-Biofilms
Approaches
Anti-biofilm approaches have to put
a stop to more than one antimicrobial
mechanism concurrently to be effective
clinically, as due to the heterogeneity
of biofilms an antibiotic or antimicrobial
agent may be able to destroy some
of the bacterial cells present in
a biofilm, but it is very improbable
for it to efficiently kill them all.
All the currently used antibiotics
were developed on account of their
activity against bacterial cultures
growing in a planktonic environment.
Thus, development of new screens of
currently accessible and potential
antibiotics might yield clinically
effective antimicrobial agents against
biofilm infections if they are selected
for their activity against biofilm
or non-growing cells. As genes and
the products of genes responsible
for antibiotic resistance of biofilm
are identified and characterized,
these will become chemotherapeutic
targets to augment the effectiveness
of currently in use antibiotics against
infections caused by biofilms.
As the antibiotic resistance of a
biofilm depends on multicellular communities
of bacteria, one of the anti-biofilm
strategies may be development of therapies
that can disrupt this multicellularity
of the biofilm. If that happens, defenses
of the host will be able to resolve
the infection, and the effectiveness
of antibiotic agents may be enhanced
(Stewart and Costerton, 2001).
Some of the other potential antibiofilm
strategies include QSIs and improvement
of drug delivery systems which are
explained in the sections below.
4.1. Use of Quorum Sensing Inhibitors
There exists in nature several compounds
that can act as Quorum Sensing Inhibitors
(QSI). A case of such a naturally
existing QSI is found to be present
in garlic extract, which renders otherwise
resistant biofilms liable to antibiotic
therapy both in vivo and in vitro.
The naturally occurring QSI compounds
can also be synthesized and subjected
to structural modification for inhibition
of QS in experimental animal infections
(in vivo). Since it is well known
that the bacteria present in a biofilm
lead to chronic infections due to
their communication, QSIs can be used
to cure these infections. Some antibiotics
such as azithromycin, ceftazidime
and ciprofloxacin inhibit QS in Pseudomonas
aeruginosa leading to inhibition of
the bacterial virulence. Thus QSIs
have improved the synergistic weak
effects of antibiotics on bacterial
biofilms leading to elimination of
biofilms. Current research states
that resistance against QSI can only
occur due to mutations (Hoiby et al.
2010). If this holds true, then conventional
problems of antibiotic resistance
that we face nowadays will not be
a clinical problem.
4.2. Improvement of Drug Delivery
System
Numbers of strategies have been proposed
for prevention of biofilm formation
and colonization, drug accumulation
at the surface of biofilm and its
delivery into the biofilm. An effective
approach to counter antibiotic resistance
of biofilms is the use of a better
or more effective drug delivery system
which could be done by the processes
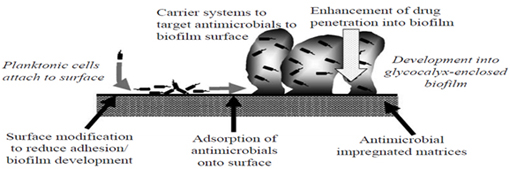
illustrated in Figure 5 (Smith, 2005).
Figure 5. Antibiofilm strategies
(Source: Smith, 2005)
4.2.1. Liposomal Delivery to Biofilms
Liposomes are considered as attractive
vehicles for drug targeting/delivery
because of their compatibility with
biological components. In the context
of infection treatment via antibiotics
liposomes have been studied for their
ability to deliver these antibiotic
agents into the biofilm. Research
shows that if a drug is targeted to
the biofilm by anionic liposomes,
each of the bacteria in the biofilm
adsorbs the drug separately to a smaller
extent. Interestingly when it targets
mixed biofilms then the drug is most
effective against one but ineffective
against the other, for example when
liposomes laden with the bactericide
triclosan targeted mixed biofilms
of Streptococcus salivarius and Streptococcus
sanguis, they were most useful against
Streptococcus sanguis, but relatively
useless against Streptococcus salivarius
(Robinson et al. 2001).
5. Conclusion
Antibiotic resistance of biofilms
depends not only upon the bacterial
colony of the biofilm but also to
the antibiotic agent used to destroy
that biofilm along with inherent characteristics
of biofilms including their mode of
growth, environmental heterogeneity
leading to the generation of heterogeneous
population, signaling, mutations etc.
Nonetheless, further studies are required
for further elucidation of how the
bacterial biofilms protect themselves
against microbial agents. Additional
cause of antibiotic resistance of
biofilms is that almost all the currently
available antibiotics have shown to
be less effective for treating biofilms
due mainly to the reason that they
were designed on account of their
activity against planktonic cells
in conventional laboratory culture
and have a small range of cellular
targets. In the meanwhile eradication
of bacterial biofilm depends on combined
treatment strategies, quorum sensing
inhibition and modern drug delivery
approaches.
6. References
Alhede, M., Bjarnsholt, T., Jensen,
P. O., Phipps, R. K., Moser, C., and
Christophersen, L. (2009). Pseudomonas
aeruginosa recognizes and responds
aggressively to the presence of polymorphonuclear
leukocytes. Microbiology, 155, 3500-3508.
Bjarnsholt, T., Kirketerp-Moller,
K., Kristiansen, S., Phipps, R., Nielsen,
A. K., and Jensen, P. O. (2007). Silver
against Pseudomonas aeruginosa biofilms.
Acta Pathologica Microbiologica et
Immunologica Scandinavica,115, 921-928.
Boaretti, M. (2003). Involvement of
rpoS in the survival of Escherichia
coli in the viable but non-culturable
state. Environmental Microbiology,
5, 986-996.
Conibear, T. C., Collins, S. L., and
Webb, J. S. (2009). Role of mutation
in Pseudomonas aeruginosa biofilm
development. PLoS One, 14,62-89.
Cunningham, A. B., Lennox, J. E.,
and Ross, R. J. (2008). Introduction
to Biofilms: What are their characteristics?
Retrieved 4th January, 2014 from:
http://biofilmbook.hypertextbookshop.com.
Donlan, R. M. (2002). Biofilms: Microbial
life on surfaces. Emerging Infectious
Diseases Journal, 8, 881-890.
Driffield, K., Miller, K., Bostock,
M., O'Neill, A. J., and Chopra, I.
(2008). Increased mutability of Pseudomonas
aeruginosa in biofilms. Journal of
Antimicrobial Chemotherapy, 61,1053-1056.
Evans, R.C. and Holmes, C.J. (1987).
Effect of vancomycin hydrochloride
on Staphylococcus epidermidis biofilm
associated with silicone elastomer.
Antimicrobial Agents Chemother, 31,
889-894.
Gennip, M. V., Christensen, L. D.,
Alhede, M., Phipps, R., Jensen, P.
O., and Christophersen, L. (2009).
Inactivation of the rhl A gene in
Pseudomonas aeruginosa prevents rhamnolipid
production, disabling the protection
against polymorphonuclear leukocytes.
Acta Pathologica Microbiologica et
Immunologica Scandinavica, 117,537-546.
Harrison, F. (2007). Microbial ecology
of the cystic fibrosis lung. Microbiology,
153, 917-923.
Hoiby, N., Bjarnsholt, T., Givskov,
M., Molinc, S. and Ciofub, O. (2010).
Antibiotic resistance of bacterial
biofilms. International Journal of
Antimicrobial Agents, 35, 322-332.
Jensen, P. O., Bjarnsholt, T., Phipps,
R., Rasmussen, T. B., Calum, H., and
Christoffersen, L. (2007). Rapid necrotic
killing of polymorphonuclear leukocytes
is caused by quorum-sensing-controlled
production of rhamnolipid by Pseudomonas
aeruginosa. Microbiology,153,1329-1338.
Landry, R. M., An, D., Hupp, J. T.,
Singh, P. K., Parsek, M. R. (2006).
Mucin- Pseudomonas aeruginosa interactions
promote biofilm formation and antibiotic
resistance. Molecular Microbiology,
59,142-151.
Lindsey, D. and Holy, A. V. (2006).
Bacterial biofilms within the clinical
setting: what healthcare professionals
should know. Journal of Hospital Infection,
64, 313-325.
Mah, T. C. and O'Toole, G. A. (2001).
Mechanisms of biofilm resistance to
antimicrobial agents. Trends in Microbiology,
1, 34-39.
Moskowitz, S. M., Foster, J. M., Emerson,
J., and Burns, J. L. (2004). Clinically
feasible biofilm susceptibility assay
for isolates of Pseudomonas aeruginosa
from patients with cystic fibrosis.
Journal of Clinical Microbiology,
42,1915-1922.
Robinson, A. M., Bannister, M., Creeth,
J. E., and Jones, M.N. (2001). The
interaction of phospholipid liposomes
with mixed bacterial biofilms and
their use in the delivery of bactericide,
Colloids Surface. A Physicochemical
Engineering Aspects, 186, 43-53.
Smith, A. W. (2005). Biofilms and
antibiotic therapy: Is there a role
for combating bacterial resistance
by the use of novel drug delivery
systems? Advanced Drug Delivery Reviews,
57, 1539-1550.
Stewart, P. S., and Costerton, J.
W. (2001). Antibiotic resistance of
bacteria in biofilms. Lancet,358,
135-138.
Werner, E., Roe, F., Bugnicourt, A.,
Franklin, M. J., Heydorn, A., and
Molin, S. (2004). Stratified growth
in Pseudomonas aeruginosa biofilms.
Applied Environmental Microbiology,
70, 6188-6196.
|